Written by Charlotte Zhang
Illustrated by Angela Lin
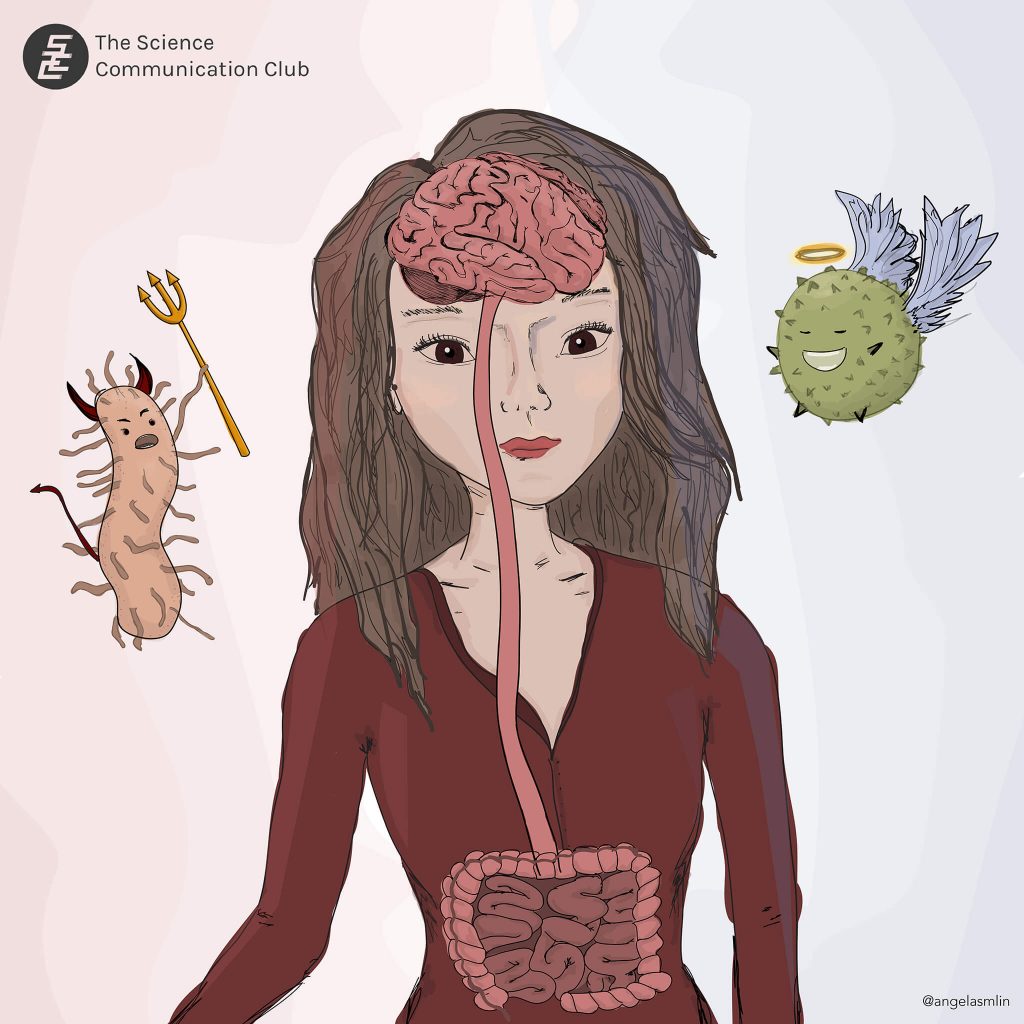
We all know that maintaining a healthy diet brings many benefits to our lives, but why does it matter so much, and what are the consequences of not doing so? In this article, we will look into an often-overlooked component in our stomach that tends to be affected by our diet: the gut microbiome, a.k.a. the composition of all the bacteria that live in the stomach.
When we talk about bacteria in the stomach, we might recall unpleasant things like E. coli, salmonella, or the many bathroom trips we took after eating that one suspicious yet delicious takeout. Would life be better without these microorganisms in our gut? The simple answer is no. Not all bacteria are harmful, and their collective existence in the gut is actually important for our survival. For example, A.muciniphila strengthen the protective lining in the gut, and A.equolifasciens enhance antioxidant effects that are important in anti-aging and cancer prevention. The gut microbiome keeps many body processes in check, including metabolism, digestion, and immune processes. Moreover, the gut microbiota can actually send and receive signals to and from our brain (known as the gut-brain axis), affecting how the brain functions. This gut-brain crosstalk influences the types of substances that are produced in our brain and indirectly affects how we think (Collins et al., 2012).
However, not all bacteria can live together in harmony, and alterations in the composition of the gut microbiome — referred to as dysbiosis — mean bad news for our health. An inappropriately structured microbiome composition may send signals to the rest of the body resulting in negative consequences. Evidence from studies suggests that dysbiosis may cause inflammation in the body and the brain. Maladaptive inflammation might further lead to seemingly unrelated disorders like depression, anxiety, and even dementia. Recent studies have found that dysbiosis caused accumulation of harmful proteins called beta-amyloids in mouse brains, which is a key indicator of the presence of Alzheimer’s Disease and other forms of dementia (Collins et al., 2012). Interestingly, after transplanting excrements (which contained healthy gut bacteria) from healthy mice to the Alzheimer’s mice, memory and motor impairment were significantly alleviated (Kim et al., 2020). As gross as it might sound, administering feces extractions to patients (known as fecal microbiota transplantation) with various disorders have shown promising results in recent clinical trials (Fong et al., 2020).

Getting rid of the gut microbiome altogether through antibiotics also creates interesting consequences. A recent study found that mice who had their microbiome erased by antibiotics developed disturbances in their sleep/wake patterns (Ogawa et al., 2020). Another study found that the depletion of our own gut microbiome weakens our liver’s immunity against viruses (Guo et al., 2021). In some cases, however, getting rid of the gut microbiome temporarily actually provides therapeutic benefits, because it allows the bacteria to “reboot” and restore a healthier balance. Studies have found that getting rid of the gut microbiome improved glucose intolerance and bile metabolism in obese hamsters (Sun et al., 2019).
Research interest in the gut microbiome continues to increase as more and more studies show promising evidence towards its importance in health maintenance and disease control. Fecal microbiota transplantation has also shown its potential in treating a variety of diseases. Applying this knowledge in our daily lives, we obviously shouldn’t go as far as looking for feces with healthy bacteria, but it is important to eat a healthy and balanced diet to keep our gut bacteria in check. Obtaining probiotics from diet (the most ideal choice) or supplements on a regular basis also helps with keeping this balance. Fruits and vegetables are good sources of fiber, and studies have found that fruit fibers can increase the growth of Bifidobacterium, which has been shown to prevent inflammation and enhance gut health (Li et al., 2020). Moreover, fermented foods like yogurt and kimchi also enhance the gut microbiome. Consumption of yogurt fortified with Lactobacilli and Bifidobacterium can even reduce lactose intolerance in some individuals (Masoumi et al., 2021). Overall, the gut microbiome is an essential part of our body that should be paid more attention to regarding our personal health.
Keywords: Gut microbiome, gut-brain axis, inflammation, personal health
Sources:
- Collins, S. M., Surette, M., & Bercik, P. (2012). The interplay between the intestinal microbiota and the brain. Nature Reviews. Microbiology, 10(11), 735–742.
- Fong, W., Li, Q., & Yu, J. (2020). Gut microbiota modulation: a novel strategy for prevention and treatment of colorectal cancer. Oncogene, 39(26), 4925–4943.
- Guo, W., Zhou, X., Li, X., Zhu, Q., Peng, J., Zhu, B., Zheng, X., Lu, Y., Yang, D., Wang, B., & Wang, J. (2021). Depletion of gut Microbiota impairs gut barrier function and antiviral immune defense in the liver. Frontiers in Immunology, 12, 636803.
- Kim, M.-S., Kim, Y., Choi, H., Kim, W., Park, S., Lee, D., Kim, D. K., Kim, H. J., Choi, H., Hyun, D.-W., Lee, J.-Y., Choi, E. Y., Lee, D.-S., Bae, J.-W., & Mook-Jung, I. (2020). Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut, 69(2), 283–294.
- Li, Y., Wang, S., Sun, Y., Zheng, H., Tang, Y., Gao, X., Song, C., Liu, J., Long, Y., Liu, L., & Mei, Q. (2020). Apple polysaccharide could promote the growth of Bifidobacterium longum. International Journal of Biological Macromolecules, 152, 1186–1193.
- Masoumi, S. J., Mehrabani, D., Saberifiroozi, M., Fattahi, M. R., Moradi, F., & Najafi, M. (2021). The effect of yogurt fortified with Lactobacillus acidophilus and Bifidobacterium sp. probiotic in patients with lactose intolerance. Food Science & Nutrition, 9(3), 1704–1711.
- Ogawa, Y., Miyoshi, C., Obana, N., Yajima, K., Hotta-Hirashima, N., Ikkyu, A., Kanno, S., Soga, T., Fukuda, S., & Yanagisawa, M. (2020). Gut microbiota depletion by chronic antibiotic treatment alters the sleep/wake architecture and sleep EEG power spectra in mice. Scientific Reports, 10(1), 19554.
- Sun, L., Pang, Y., Wang, X., Wu, Q., Liu, H., Liu, B., Liu, G., Ye, M., Kong, W., & Jiang, C. (2019). Ablation of gut microbiota alleviates obesity-induced hepatic steatosis and glucose intolerance by modulating bile acid metabolism in hamsters. Acta Pharmaceutica Sinica. B, 9(4), 702–710.