
Written by Anne-Marie Bulboaca
Illustrated by Sabrina Chen
In 2005, a 13 year-old boy suffering from ataxia telangiectasia presented to Sheba Medical Center, Israel, with persistent headaches. Ataxia telangiectasia is a rare hereditary neurological disorder affecting the motor and speech centers of the brain, the spine, and the immune system, for which there is currently no cure or treatment to slow progression¹. After examination and testing, doctors discovered that the boy had developed two lesions, one in his brain and the other in his spinal cord. They were able to perform surgery to remove the masses, and they used genetic analysis to characterize the cells. Strangely, the mass had high levels of X chromosome presence, suggesting it had a female origin, and it contained genes that could not possibly have originated from the boy or his parents². So where did these mysterious cells come from?
In 2001, 2002, and 2004, the boy and his parents had travelled to a clinic in Russia where he received alleged fetal neural stem cell injections into his brain and spinal cord with the intention of treating his ataxia. This case was one of the first reported adverse outcomes resulting from stem cell tourism³, and represents an increasingly common phenomenon occurring across the world.
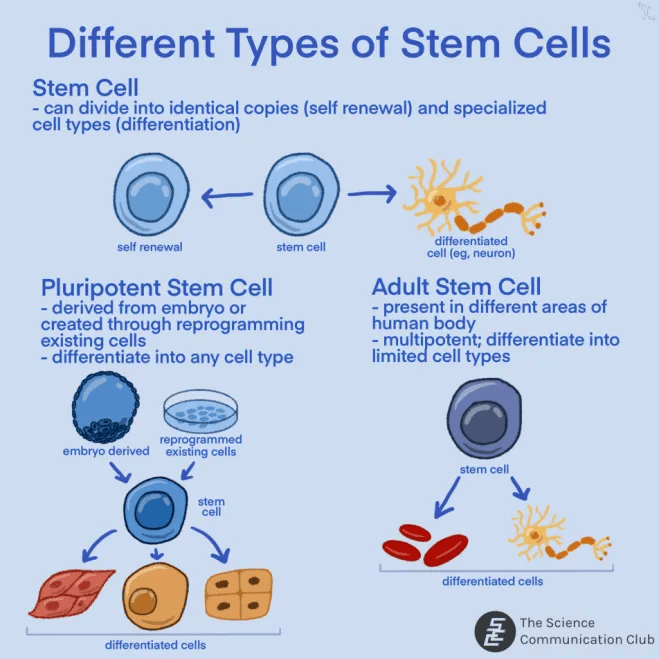
Stem cell research has been making headlines highlighting its development of potential new treatments for a wide range of diseases. Stem cells have two key abilities: they can make exact copies of themselves (self-renewal) and develop into specialized cells (differentiation), which allows them to replace damaged tissues. There are many types of stem cells arising from a range of sources. Pluripotent stem cells can differentiate into almost any cell type in the body, and must be derived from an embryo or created through the reprogramming of existing cells. Adult stem cells are present in various locations in the adult human body, and are usually multipotent, meaning they can differentiate into a limited number of specific cell types⁴. As of December 2024, there were over 100 active clinical trials studying stem cell-based treatments for cancer, Parkinson’s disease, diabetes, epilepsy, heart failure, and eye diseases⁵. It is undeniable that stem cells have incredible potential for innovation in the healthcare field.
However, clinics offering unproven stem cell interventions (SCIs) have exploited this potential, selling stem cell products that have little to no evidence supporting their safety or efficacy. Stem cell tourism is the phenomenon of individuals travelling internationally to receive these SCIs. It began in the early 2000s with clinics appearing around the world, usually in countries with loosely regulated healthcare and pharmaceutical industries. However, a concerning trend shows these clinics popping up across Europe, North America, Australia, and Southeast Asia, where regulations are far more robust⁶. In fact, the United States now has more businesses selling SCIs than countries that were top destinations for stem cell tourism. As of March 2021, there were 1,480 businesses operating 2,754 clinics selling SCIs in the US. Interventions included a wide variety of stem cell types. Concerningly, approximately 15% of clinics were advertising SCIs without listing their source or the type of stem cells used. Only around 4% of clinics listed the costs of their treatments, which are generally incredibly expensive⁷.
Clinics offering SCIs use a direct-to-consumer model, advertising over the internet⁸. They often claim to be able to treat life-altering or deadly conditions with no existing cure, including chronic pain, orthopedic conditions, neurological conditions, immunological diseases, lung and respiratory conditions, and cardiovascular diseases. They may even target minors and their parents, marketing treatments for childhood diseases such as Cerebral Palsy⁷. SCI products are not FDA approved, and clinics often cite fake scientific articles or use animal studies to support their claims⁶. They advertise patient satisfaction as high as 100%⁹, and highlight patient testimonials that present glowing reviews of miraculous cures. Previous patients are often monetarily incentivized to provide these reviews¹⁰. These predatory advertising tactics exploit desperate people suffering from drastic diseases and give them false hope of recovery.
There have been many highly reported cases of adverse effects caused by SCIs, including multiple class action lawsuits against US clinics offering these treatments. In 2016, a man developed a spinal cord lesion after spending close to $300,000 to receive stem cell injections at clinics in China, Argentina, and Mexico¹⁰. In 2017, three elderly women experienced vision loss and retinal detachment after receiving stem cell injections in both eyes as part of a fake clinical trial aiming to treat age-related macular degeneration¹¹. Highly publicized cases like these erode public trust in the healthcare industry and scientific research.
In 2022, 1063 former patients of California-based clinic StemGenex received a settlement of $3.65 million in a class action lawsuit. The clinic has claimed that its treatment was effective against Alzheimer’s, Parkinson’s, multiple sclerosis, lupus, and diabetes. They charged $14,900 per treatment and advertised 100% customer satisfaction. However, none of the plaintiffs saw any significant benefit from the treatments they received⁹. Cases like this show that these clinics can face consequences, and experts hope that lawsuits will shift the tide of SCIs by raising awareness about the dangers of the industry and increasing patient advocacy⁸.
While stem cell tourism and SCIs present significant risks, they also highlight the urgent need for public education and continued research. As science advances, the potential for safe, effective stem cell therapies to revolutionize medicine is bright, offering hope for patients in need of transformative care.
Sources:
- Ataxia Telangiectasia | National Institute of Neurological Disorders and Stroke. [accessed 2025 Feb 28]. https://www.ninds.nih.gov/health-information/disorders/ataxia-telangiectasia
- Amariglio N et al. Donor-Derived Brain Tumor Following Neural Stem Cell Transplantation in an Ataxia Telangiectasia Patient. PLOS Medicine. 2009;6(2):e1000029. https://doi.org/10.1371/journal.pmed.1000029
- Bauer G, Elsallab M, Abou-El-Enein M. Concise Review: A Comprehensive Analysis of Reported Adverse Events in Patients Receiving Unproven Stem Cell-Based Interventions. Stem Cells Translational Medicine. 2018;7(9):676–685. https://doi.org/10.1002/sctm.17-0282
- Stem Cell Basics | STEM Cell Information. [accessed 2025 Feb 28]. https://stemcells.nih.gov/info/basics/stc-basics
- Abbott A. Stem cells head to the clinic: treatments for cancer, diabetes and Parkinson’s disease could soon be here. Nature. 2024;637(8044):18–20. https://doi.org/10.1038/d41586-024-04160-0
- Master Z, Matthews KRW, Abou-el-Enein M. Unproven stem cell interventions: A global public health problem requiring global deliberation. Stem Cell Reports. 2021;16(6):1435–1445. https://doi.org/10.1016/j.stemcr.2021.05.004
- Turner L. The American stem cell sell in 2021: U.S. businesses selling unlicensed and unproven stem cell interventions. Cell Stem Cell. 2021;28(11):1891–1895. https://doi.org/10.1016/j.stem.2021.10.008
- Horner C, Tenenbaum E, Sipp D, Master Z. Can civil lawsuits stem the tide of direct-to-consumer marketing of unproven stem cell interventions. npj Regenerative Medicine. 2018;3(1):1–5. https://doi.org/10.1038/s41536-018-0043-6
- X, Email, Facebook, Bluesky. Column: A stem cell clinic and its doctor will pay a $3.65-million settlement to 1,100 ex-patients. Los Angeles Times. 2022 Mar 10 [accessed 2025 Feb 27]. https://www.latimes.com/business/story/2022-03-10/stemgenex-stem-cell-settlement
- Kolata G. A Cautionary Tale of ‘Stem Cell Tourism.’ The New York Times. 2016 Jun 23 [accessed 2025 Feb 28]. https://www.nytimes.com/2016/06/23/health/a-cautionary-tale-of-stem-cell-tourism.html
- Kuriyan AE et al. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. The New England Journal of Medicine. 2017;376(11):1047–1053. https://doi.org/10.1056/NEJMoa1609583