
Written by Jameson Brown
Illustrated by Cheryl Nong
There are many vertebrates (animals with a backbone) on Earth, including humans, that have lost their limbs due to trauma. All of these vertebrates must learn to live without these limbs or use a prosthetic solution. Now, imagine if these limbs could grow back. For the salamander, we do not have to imagine. Adult salamanders are the only vertebrates that can regenerate their limbs and tails throughout their life¹.
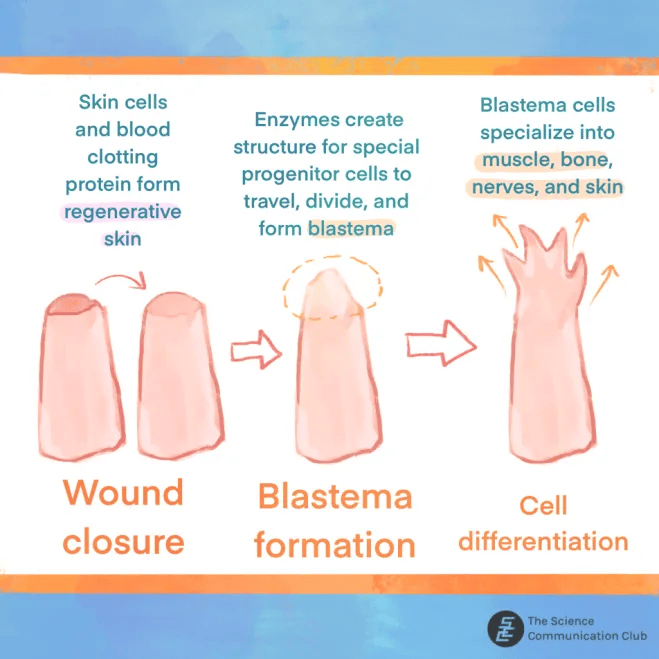
How are salamanders able to regenerate their limbs? The answer is three major events that occur during limb regeneration in the salamander: wound closure, formation of the blastema, and cell differentiation mechanisms¹. Wound closure begins when keratinocytes, cells that form the outermost layer of the skin, and fibrin, a molecule that is involved in blood clotting, join to form the regenerative skin¹,²,³. Next, enzymes (proteins that speed up chemical reactions in the body) called extracellular matrix metalloproteinases (MMPs) form a structure that allows mesenchymal cells (cells that can develop into bone-building cells, bone-breaking cells, and cartilage cells) to pass through and divide into progenitor cells (cells that can develop into a specialized cell), the blastema¹,⁴. The blastema is made up of cells from many different tissues, including muscle, bone, nerve, and skin that are needed to regenerate a limb¹. The cells then begin to grow, divide, and reproduce, leading to the final product: a new limb ¹,²,³.
Claudia Arenas-Gómez and Jean-Paul Delgado from the Universidad de Antioquia suggest that salamanders can sustain their regenerative capacity throughout their life because their immune system is underdeveloped¹. As an embryo, mammals (animals characterized by their ability to produce milk with fur or hair covering the skin) can form regenerative processes with an immature immune system. However, as the mammal’s immune system matures, they lose their ability to regenerate tissues, limbs, and organs¹. Despite that, cells involved in the immune response, such as macrophages (a type of white blood cell that stimulates the activity of other immune system cells and kills bacteria), are important in limb regeneration. When researchers prevented these cells from functioning, limb regeneration did not occur¹,⁵.
The salamander model for limb regeneration has contributed to research in regenerative medicine, a field of medicine that uses engineering and life science principles to aid in regeneration of diseased and injured tissues or organs⁶. Regenerative medicine scientists are particularly interested in one aspect of the salamander limb regeneration process: the blastema. The blastema can be removed from the stump of the regenerating limb and placed in another location⁷. However, the blastema will still develop into the limb where it originated. Jeremy Brockes and Phillip Gates from University College London, suggest that this discovery emphasizes a crucial direction in constructing mammalian blastema as this group of cells can generate a limb without intrusive intervention⁷. Although we are not yet able to regenerate our limbs, with the help of the salamander regeneration process, we may be one step closer.
Sources:
- Arenas-Gómez C.M. & Delgado J. Limb regeneration in salamanders: the plethodontid tale. J. Dev. Biol. 2021;65, 313-321. doi: 10.1387/ijdb.200228jd
- National Library of Medicine. Keratinocytes. https://www.ncbi.nlm.nih.gov/mesh?Db=mesh&Cmd=DetailsSearch&Term=%22Keratinocytes%22%5BMeSH+Terms%5D
- Litvinov R.I. & Weisel J.W. What Is the Biological and Clinical Relevance of Fibrin? Thromb. Hemost. 2016; 42(4), 333-343. doi: 10.1055/s-0036-1571342
- Uccelli A., Moretta L., & Pistoia V. Mesenchymal stem cells in health and disease. Rev. Immunol. 2008;8, 726-736. doi: 10.1038/nri2395
- National Cancer Institute. Macrophage. https://www.cancer.gov/publications/dictionaries/cancer-terms/def/macrophage
- Mao A.S. & Mooney D.J. Regenerative medicine: Current therapies and future directions. Natl. Acad. Sci. U S A. 2015;112(47), 14452-14459. doi: 10.1073/pnas.1508520112
- Brockes J.P. & Gates P.B. Mechanisms underlying vertebrate limb regeneration: lessons from the salamander. Soc. Trans. 2014;42(3), 625-630. doi: 10.1042/BST20140002