Written by Rish Johri
Illustrated by Amelia Han
Topics: Depression and Mental Health, Immunology, Gut-Brain axis
Depression, or major depressive disorder (MDD), is one of the most prevalent mental health conditions, affecting around 300 million people globally¹. Depression occurs about twice as often in women than in men and primarily affects people between 18 and 25 years old but can occur at any age. A particularly concerning trend is that the prevalence of depression is rapidly increasing. In 2018, the World Health Organization ranked MDD third by disease burden. By 2030, it is predicted to rank first². More and more people are being diagnosed with depression or mental illnesses associated with depression and at increasingly younger ages too. MDD is commonly treated using a class of drugs known as Selective Serotonin Reuptake Inhibitors (SSRIs). However, these drugs are far from being a magical cure. SSRIs do not work for approximately 30 percent of people with major depression, even after multiple treatment courses have been tried. This discouraging statistic, along with frequent reports of adverse effects from first-line depression treatments, has prompted a surge of research into the causes underlying depression to improve diagnosis and treatment³. Many risk factors have been attributed to the onset of depression, including genetics, epigenetics, social-psychological factors, and comorbidities¹. Recently, a new theory has been developed known as the “inflammatory hypothesis”, the idea that depression is associated with abnormal immune responses.
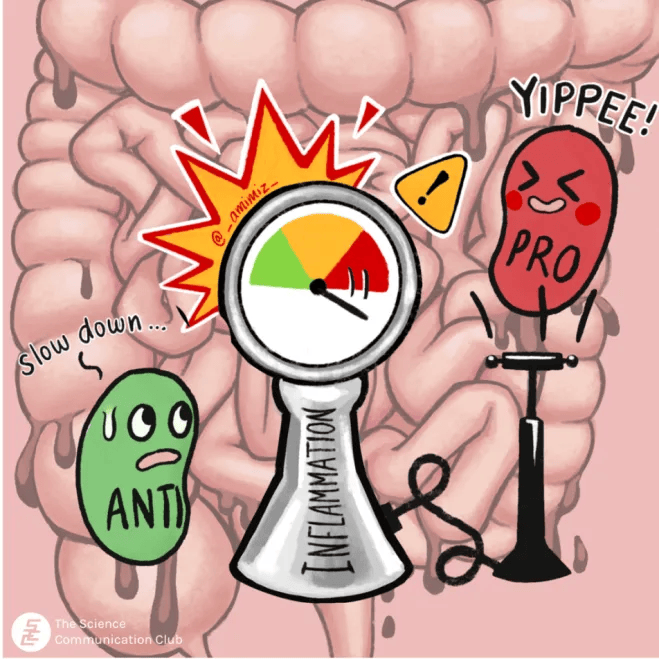
The inflammatory hypothesis may be relatively new, but the earliest link between depression and the immune system was established in the 1930s, when Hans Selye’s Stress Adaptation Theory described the shrinkage of “immune organs” like the thymus, spleen and lymphoid tissues in adverse mental conditions⁴. Since then, immune dysregulation has been consistently associated with psychiatric disorders, and depression has been associated with chronic physical and autoimmune diseases like coronary heart disease, cancer, and diabetes. This bi-directional relationship between depression and immunity has sparked investigation into the immune-mediated mechanisms behind this connection. Compelling evidence has suggested that cytokines, the immune system’s signaling molecules, may play a role in depressive behaviour⁵. Pro-inflammatory cytokines that communicate danger signals and amplify immune responses have been shown to circulate at higher levels in depressed individuals. At the same time, anti-inflammatory cytokines are found at lower levels and regulatory pathways that dampen excessive inflammation are downregulated⁶.
Signaling molecules aren’t the only players in the link between depression and immunity. Preclinical animal models of depression have revealed that immune cells are heavily involved in the onset and development of depression. Th17 cells are specialized pro-inflammatory immune cells normally involved in protective immune responses against infection and injury. However, the presence of these cells can be harmful, with high levels seen in autoimmune diseases like multiple sclerosis, psoriasis, and arthritis⁷. Th17 cells are found at a higher frequency in people with MDD, and people with a high risk of suicide show a significant increase in the number of these cells in their blood⁷. In addition to Th17 cells, regulatory T cells, or Tregs are suspected to play a role in depression. Unlike Th17 cells, Tregs play crucial roles in dampening inflammation and preventing immune responses from getting out of control. A 2012 study showed that the depletion of Tregs from mice can induce anxiety- and depression-like behaviours⁸. Similar findings have been observed in humans, where people with MDD had fewer Tregs compared to healthy individuals⁹. Interestingly, MDD patients treated with antidepressants had higher Treg counts¹⁰. Together, these studies illustrate the importance of regulation in our immune system and the balance of pro- and anti-inflammatory cells.
An intriguing discovery is that the treatments we use to modulate the immune system can also alter mood. Pro-inflammatory drugs used to treat hepatitis C and cancer have been associated with increases in depression, often occurring within days¹¹. Another intriguing possibility is the “leaky gut” hypothesis, which suggests that gut inflammation and the accumulation of neurotoxic substances in the GI tract may be connected. As the gut becomes more permeable, bacteria and their secreted toxins can enter the bloodstream. Recent studies have shown that this process can trigger cytokine production and send immune signals to the brain that affect mood¹². Emerging evidence has also identified differences between the gut microbial composition of depressed and non-depressed individuals, adding a new piece to the puzzle¹³.
So, what does all of this mean? While these recent findings may not direct us toward an immediate cure, they greatly improve our understanding of depression on a molecular level. This foundational research has opened up promising avenues in immunology for developing new treatments and personalized interventions that could be key to treating depression. As we search for new pharmacologic treatments for MDD, immunotherapy could play a crucial role in fighting depression.
Sources:
- Cui, L. et al. Major depressive disorder: hypothesis, mechanism, prevention and treatment. Signal Transduction and Targeted Therapy 9, 30 (2024).
- Malhi, G.S. & Mann, J.J. Depression. Lancet 392, 2299-2312 (2018).
- Namiot, E.D. et al. Depression clinical trials worldwide: a systematic analysis of the ICTRP and comparison with ClinicalTrials.gov. Transl Psychiatry 14, 315 (2024).
- Kopin, I.J., Eisenhofer, G. & Goldstein, D. Sympathoadrenal medullary system and stress. Adv Exp Med Biol 245, 11-23 (1988).
- Felger, J.C. & Lotrich, F.E. Inflammatory cytokines in depression: neurobiological mechanisms and therapeutic implications. Neuroscience 246, 199-229 (2013).
- Paul, E.R. et al. Peripheral and central kynurenine pathway abnormalities in major depression. Brain. Behav. Immun. 101, 136-145 (2022).
- Schiweck, C. et al. Depression and suicidality: A link to premature T helper cell aging and increased Th17 cells. Brain, Behavior, and Immunity 87, 603-609 (2020).
- Kim, S.J. et al. CD4+CD25+ regulatory T cell depletion modulates anxiety and depression-like behaviors in mice. PLoS One 7, e42054 (2012).
- Grosse, L. et al. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain. Behav. Immun. 54, 38-44 (2016).
- Mohd Ashari, N.S. et al. Major depressive disorder patients on antidepressant treatments display higher number of regulatory T cells. Malays. J. Pathol. 41, 169-176 (2019).
- Capuron, L., Ravaud, A., Miller, A.H. & Dantzer, R. Baseline mood and psychosocial characteristics of patients developing depressive symptoms during interleukin-2 and/or interferon-alpha cancer therapy. Brain. Behav. Immun. 18, 205-213 (2004).
- Sanada, K. et al. Gut microbiota and major depressive disorder: A systematic review and meta-analysis. J. Affect. Disord. 266, 1-13 (2020).
- Radjabzadeh, D. et al. Gut microbiome-wide association study of depressive symptoms. Nature Communications 13, 7128 (2022).